Gas Elimination Solutions
Efficient systems for safe removal of volatile gases from well water.
Aeration VS. Ion Exchange
Aeration of Volatile Gases brings water and air in close contact in order to remove dissolved gases (such as carbon dioxide) and oxidizes dissolved metals such as iron, hydrogen sulfide, and volatile organic chemicals (VOCs). Aeration is often the first major process at the treatment plant. During aeration, constituents are removed or modified before they can interfere with the treatment processes. Aeration brings water and air in close contact by exposing drops or thin sheets of water to the air or by introducing small bubbles of air (the smaller the bubble, the better) and letting them rise through the water. The scrubbing process caused by the turbulence of aeration physically removes dissolved gases from solution and allows them to escape into the surrounding air. Aeration also helps remove dissolved metals through oxidation, the chemical combination of oxygen from the air with certain undesirable metals in the water. Once oxidized, these chemicals fall out of solution and become particles in the water and can be removed by filtration or flotation. The efficiency of aeration depends on the amount of surface contact between air and water, which is controlled primarily by the size of the water drop or air bubble. Oxygen is added to water through aeration and can increase the palpability of water by removing the flat taste. The amount of oxygen the water can hold depends primarily on the temperature of the water. (The colder the water, the more oxygen the water can hold.)
Aeration directly addresses the nature of the contaminants. Since methane and hydrogen sulfide are dissolved gases, the physical process of air stripping is the most direct and efficient method for their removal.Cost-effective and low maintenance for gas removal. Aeration systems, which operate without costly regeneration chemicals, can offer a more natural and cost-effective long-term solution for removing dissolved gases.Versatile for multiple water quality issues. Aeration not only removes gases but also effectively oxidizes dissolved metals like iron and manganese, allowing them to be easily filtered out in a combined system.Higher efficiency for methane. Aeration is the only water treatment method proven to eliminate dissolved methane gas. Ion exchange is not effective for this contaminant.Reduced secondary contaminants. Aeration does not introduce additional chemical compounds, like the chloride ions from ion exchange, into the treated water. Aeration is a more effective and practical method than ion exchange for removing methane and hydrogen sulfide from water. This is because aeration is a physical process designed to remove dissolved gases, while ion exchange is a chemical process suited for removing dissolved ions.
Aeration of Volatile Gases
Gas Eliminator
Efficiently remove methane and hydrogen sulfide from well water.


Hydrogen-Sulfide (H2S)


Methane (CH4)
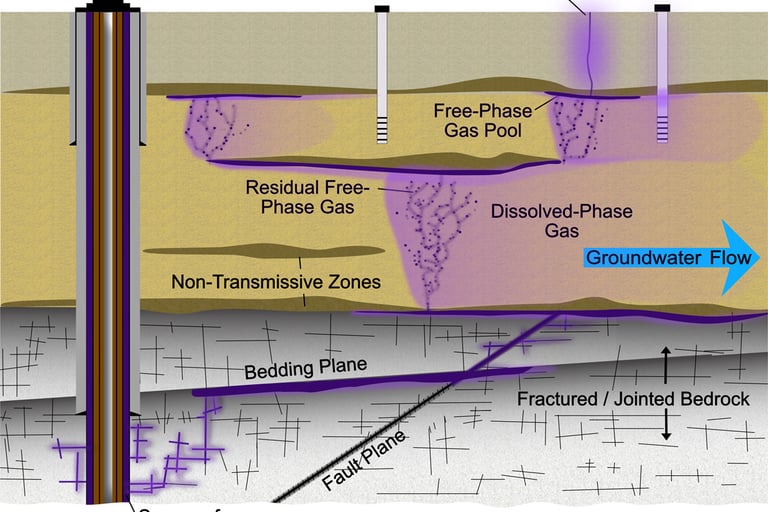
In Pennsylvania, Ohio and New York hydrogen sulfide (H2S) contamination in private water wells are a common problem, often recognized by the distinctive "rotten egg" odor it imparts to the water. This gas, though not a direct health threat, can cause nuisance issues like taste and odor problems. It's often found in wells drilled into certain types of bedrock, particularly shales and sandstones, and can also be produced by chemical reactions within water heaters or, in rare cases, by water softeners. Hydrogen sulfide is a byproduct of sulfur-reducing bacteria that thrive in low-oxygen environments like groundwater and plumbing systems. Bedrock: Wells drilled into acidic bedrock, particularly shale and sandstone formations, are more prone to hydrogen sulfide contamination. Water Heaters: The Magnesium rod used in water heaters to prevent corrosion can react with sulfates to produce hydrogen sulfide. Water Softeners: While rare, water can create conditions that encourage the growth of sulfur-reducing bacteria. Hydrogen sulfide does not cause disease or pose significant health risk at low concentrations.
Methane (CH4) in Pennsylvania, Ohio and New York water wells are a known issue, with concentrations ranging from naturally occurring biogenic sources to potential contamination from nearby natural gas extraction, particularly hydraulic fracturing (fracking). While some studies suggest methane contamination is linked to fracking, others indicate it's primarily naturally occurring, especially in areas with specific geological formations and topographic features. USGS Study: A USGS study in Lycoming County, PA, found elevated levels of methane, along with arsenic and radon, in some private wells. Orphaned oil and gas wells and closed coal mines can leak methane, a potent greenhouse gas, into the atmosphere and groundwater. Methane from these sources can contaminate well water, making it a safety hazard. The risks are exacerbated by geological factors like coal seams,, compromised oil/gas well casing damaged and infrastructure. Pennsylvania could have somewhere between 350,000 and 700,000 orphaned undocumented oil/gas wells, that will contribute to hydro-carbon gases in residential water well.